Industry News, Coatings
New Types of Silicone Resin Open Up Wider Fields of Application
Industry News, Coatings

The chemical structures of silicone resins and silicone hybrid resins result in remarkable property profiles that cannot be achieved by other classes of resins. These binders are used as the main constituent in many industrial applications, ranging from weather- and chemical-resistant coatings for building conservation to high-temperature-resistant paints.
Silicone resins are used primarily in the latter application due to their higher silicone content and their ability to withstand high temperatures better than silicone hybrid resins. High-temperature-resistant coatings – used for exhaust systems, industrial ovens, grills and combustion chambers – must retain corrosion protection and weather resistance properties, and satisfy very high demands on thermal stability. Such coatings are normally used on steel at dry film thicknesses of 20-25 µm.
Depending on their chemical structure, silicone resins can display the following special properties:
The silicone resins used in high-temperature-resistant coatings as binders in solvent, liquid resin and emulsion form are mainly methyl silicone resins and methyl-phenyl silicone resins. Silicone resins that contain only phenyl groups are used in niche applications because the resultant coatings are thermoplastic and therefore not always suitable.
Methyl silicone resins are the polymethyl siloxanes with the lowest organic content. If formulated as a nonpigmented coating, the long-term temperature resistance would be between 180 °C and 200 °C. However this is uncommon.
The temperature stability of pigmented films can be increased up to 600 °C by the addition of inorganic pigments such as aluminum flakes, mica or black iron oxide.
Long-term exposure to high temperatures usually results in complete oxidation of the methyl groups, leaving a SiO2 skeleton. This chemical analogy to silica explains the partially inorganic character of this class of resin. Commercially, methyl silicone resins are mainly distributed in solvents.
Thus, the resins retain the following characteristics of polymethyl siloxanes:
In addition to methyl groups, methyl-phenyl silicone resins generally have a phenyl content of over 20%. The phenyl groups within these resins increase the long-term heat resistance to 200-250 °C. Here too, heat resistance may (depending on the formulation) be increased to 650 °C by addition of inorganic pigments, for example.
Compatibility with organic compounds, such as resins or co-binders, is markedly improved. This improved miscibility means that methyl-phenyl silicone resins are frequently used as a starting point for the synthesis of hybrid silicone resins. However, these methyl-phenyl silicone resins are not readily miscible with methyl silicone resins because their polarities differ greatly. Methyl-phenyl silicone resins are generally commercially supplied in aromatic solvents.
Methyl silicone resins and methyl-phenyl silicone resins are usually divided into two types: the classic heat-curing systems, which are dried in ovens at high temperatures to achieve their final film properties, and the novel and versatile ambient-cure systems.
In the case of the classic heat-curing systems, physical drying occurs first, i.e. solvents escape from the paint formulation. Subsequently, applied heat initiates chemical crosslinking of the resin molecules. In contrast, the ambient-cure systems do not require the application of heat. Physical drying and chemical crosslinking occur simultaneously at room temperature.
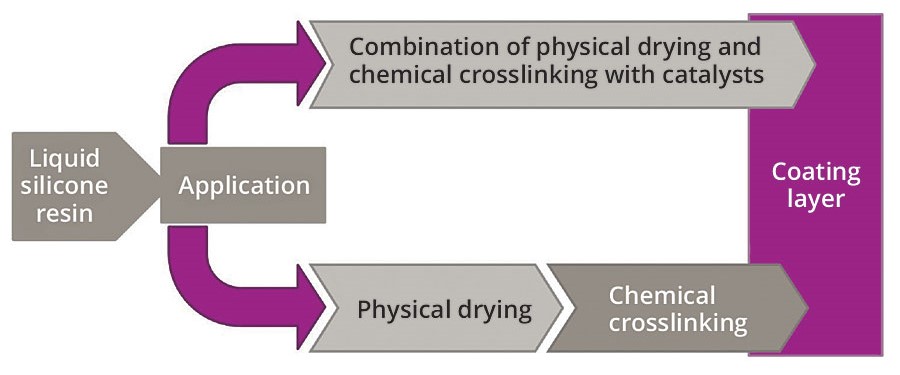
The chemical crosslinking is initiated, not by heat, but by the addition of catalysts in the presence of atmospheric moisture. The various curing conditions and processes are shown schematically in Figure 1.
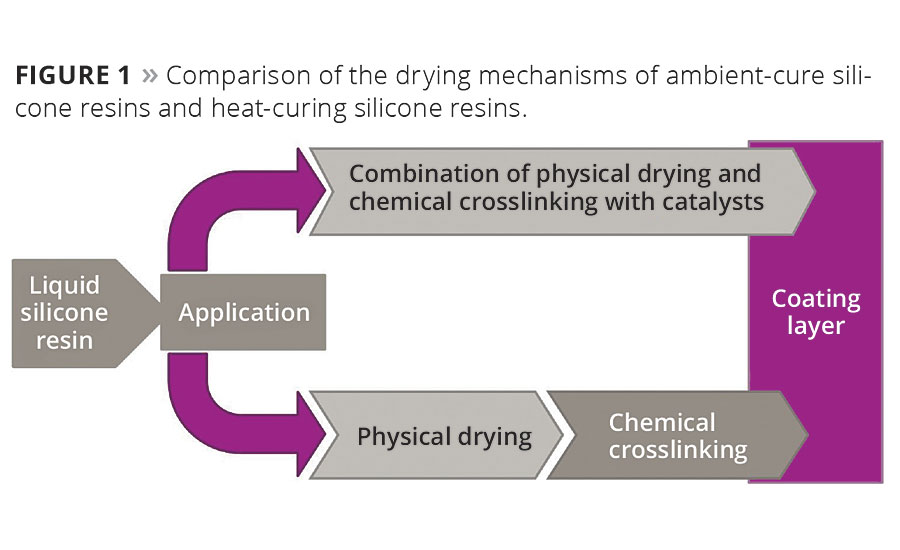
Figure 1. Comparison of the drying mechanisms of ambient-cure silicone resins and heat-curing silicone resins. © PCI
To accelerate curing of the ambient temperature-curing systems in the presence of atmospheric moisture, it is essential to add suitable catalysts such Catalyst 1 (tetra-n-butyl titanate, TnBT) or mixtures of Catalyst 1 and Catalyst 2 (tetramethylguanidine, TMG). The chemical structures of these compounds are shown in Figures 2 and 3 respectively.
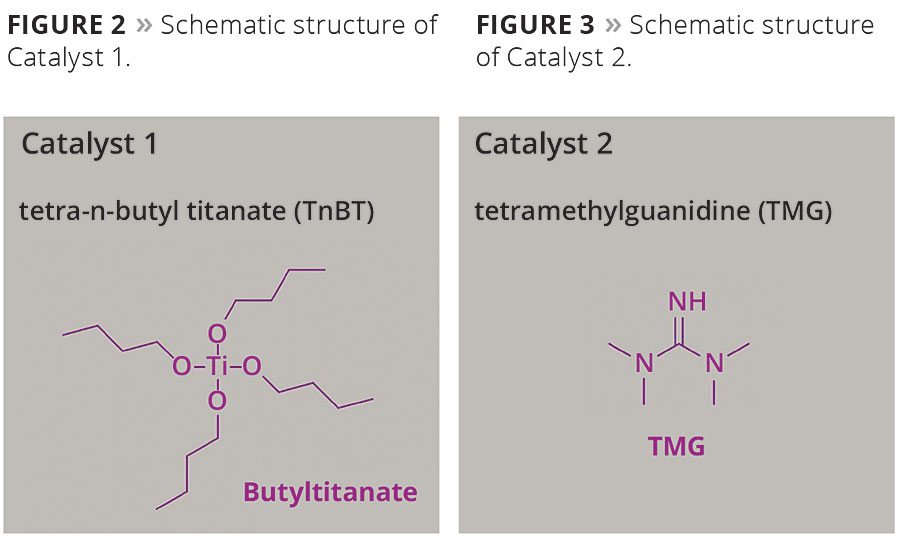
Figure 2. Schematic structure of Catalyst 1. Figure 3. Schematic structure of Catalyst 2. © PCI
In such a combination, Catalyst 1 reacts as a Lewis acid by forming a chemical bond to the polymer, and Catalyst 2 acts as a strong base accelerating the reaction rate. Both catalysts are miscible and soluble in xylene. The amounts added are 0.5-6%, relative to the solids content of the silicone resin used.
For complete crosslinking, it is important to recognize that the presence of atmospheric moisture is critical, as the water is required to hydrolyze the alkoxy group of the ambient temperature-curing silicone resin. Only after hydrolysis can condensation of the silanol groups occur.
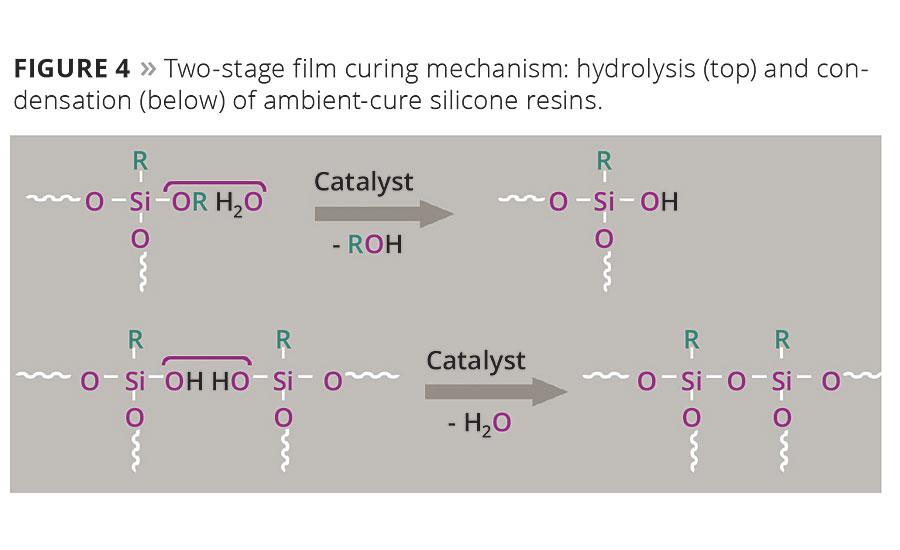
Figure 4. Two-stage film curing mechanism: hydrolysis (top) and condensation (below) of ambient-cure silicone resins. © PCI
The film-curing mechanism is thus a hydrolysis-condensation reaction (Figure 4), which requires the presence of water (atmospheric moisture) and does not require the high temperatures that are essential for traditional heat-curing systems. The decisive structural differences between the binder systems are the functionality density and the molecular weight (Figure 5).
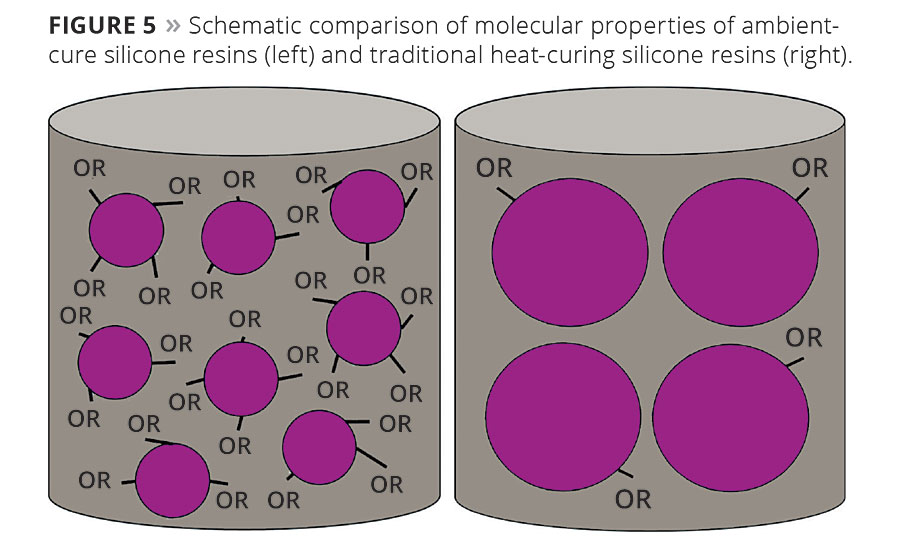
Figure 5. Schematic comparison of molecular properties of ambient-cure silicone resins (left) and traditional heat-curing silicone resins (right). © PCI
Methyl silicone resin and methyl-phenyl silicone resin stoving systems, cured at high temperatures in ovens, have significantly higher molecular weights than those of the ambient-curing silicone resins. Additionally, stoving resins also have a very low functionality density of alkoxy or silanol groups. Typically, such silicone resins are heat-cured for 30 min at approx. 250 °C to obtain a hard, completely crosslinked coating.
Ambient-curing silicone resins are high-alkoxy-functional, low-molecular-weight silicone resin elements. The low molecular weights result in products with very low viscosities, thus permitting very good processability, e.g. in spray application. Also, such systems possess a very high active substance content, which permits the formulation of high-solids coatings systems with very low VOC content.
As a rule, the alkoxy content in ambient-curing methyl silicone resins is approximately 15-30% w/w and they are commercially available with an active substance content of up to 100%.
In the field of methyl-phenyl silicone resins, the latest developments in the catalysis of the hydrolysis/condensation reactions have made large-scale curing of ambient-temperature-crosslinking silicone resins possible.
An innovative methyl-phenyl siloxane resin has a methoxy content of between 15 and 20% w/w and an active substance content of 90% (solvent: xylene). It is important to note the low viscosity of around 130 mPas, which gives the formulator a great deal of freedom, since only very low amounts of solvent may need to be added during coating manufacture. The benefit is very low smoke formation during the initial stoving.
Because of regulations in some areas of application, development of new high-solids silicone resins is based on ethoxy functional derivatives. One such resin has an ethoxy content of between 18 and 25% w/w and an active substance content of 95% (solvent: methoxypropyl acetate), with an outstandingly low viscosity of only approximately 50 mPas, which is particularly suitable for very low-solvent-content paint systems.
Generally, the cured films of the methyl-phenyl silicone resins are distinguished by very good adhesion, good flexibility and better compatibility with organic components. The methyl siloxane resin has a methoxy content of 30-40% w/w and an active substance content of 100%. Combined with an extremely low viscosity of approximately 10 mPas, this renders the addition of solvent to the formulation almost unnecessary.
Smoke generation during the first stoving process can be almost completely neglected. The hardness of the cured films is very high, and they exhibit very good color stability and strong water repellency. The advantages resulting from drying at ambient temperature are obvious. With curing at high temperatures, the size of objects to be coated is limited by the dimensions of the oven.
With ambient-curing silicone resins, even large objects (usually too large for an oven) can be coated using coatings based on high-temperature-resistant silicone resins, thus opening up further fields of application for high-temperature-resistant coatings. It should, however, be noted that large amounts of alcohol are liberated during curing of these resins.
Last, but not least, energy consumption with ambient-curing silicone resins is significantly less than with heat-curing systems.
The commercial success of silicone resins in the field of high-temperature-resistant coatings is based on their special properties. Alongside the classic heat-curing systems, ambient-curing systems are enjoying ever greater success.
These systems cure at ambient temperature using a catalyst in the presence of atmospheric moisture, thus economizing on the energy needed for stoving. The size of the parts to be coated is not limited by the size of the oven, thus opening up further fields of application, particularly in industry.
The smoke formation, which occurs with traditional heat-curing silicone resins, and the VOC contents are markedly reduced – thus meeting the continuously increasing targets for more eco-friendly coatings systems.