Industry News, Cosmetics & Persnoal Cares
Powerful Surfactants From Renewable Raw Materials

Industry News, Cosmetics & Persnoal Cares

‘Green’: This is the common prognosis regarding long-term trends for personal care products in a nutshell.
There is already an increasing interest in products based on components that are part of the human metabolism, ideally made completely from renewable raw materials and easily biodegradable. Therefore, products based on amino acids, carbohydrates and fats are in the spotlight. The ‘juicy’ task for the chemical industry is to develop personal care products with this profile but without loss in performance in comparison to powerful petrochemical products.
For Zschimmer & Schwarz this demand led to the development of surfactants which match these requirements as closely as possible. In the following we will guide you through the products that are fulfilling most or even all of the requirements mentioned above.
The ‘green’ tool box of Zschimmer & Schwarz consists of the building blocks of fats – glycerol, fatty acids and their derivatives – and amino acids including their natural derivatives. In our portfolio there are two product series which are completely based on these natural building blocks: The Protelan- and the SebumolSeries. The chemical linkages between the hydrophobic part and the hydrophilic part of the surfactant molecule in the ProtelanSeries are amide bonds, whereas in the surfactants of the Sebumol-Series they are ester bonds. Both bonds are also extensively ‘used’ by nature and can be cleaved by nature’s enzymes (peptidases and lipases). As a result all surfactants of the Protelan- and the Sebumol-Series are easily biodegradable.
The Protelan-Series includes products where fatty acids are linked with amino acids or peptides. They are produced via a two-step synthesis route (SchottenBaumann reaction). The amino acids used are glutamate, sarcosinate and glycinate, the peptide is hydrolysed wheat protein.
Long-chain acyl glutamates are extraordinarily mild and skin-friendly. This is the reason for using them especially in body cleansing formulations. In combination with other surfactants the performance is significantly enhanced by adding acyl glutamates if foam volume or foam stability is not sufficient. Apart from that, acyl glutamates are suitable as powerful emulsifiers for cold-processable oil-in-water emulsions including a wide range of different polar oils. They provide a very light and velvety skin feeling.
The acyl glutamates produced by Zschimmer & Schwarz are the AG-Types. The glutamate used is produced via a biotechnological route (fermentation) starting with glucose or its polymers. All AG-Types are clear aqueous solutions and preservative-free.
The acyl glutamates in AGL 95 and AGL 95/C are derivatives of lauric acid and coconut fatty acid respectively. Due to the manufacturing process both products contain small amounts of propylene glycol. A few years ago an important step in the direction of ‘green’ was taken by the development of a cocoyl glutamate solution – in which propylene glycol is replaced by 1,3-propanediol (AGE). The latter is made from corn starch. AGE is therefore completely based on renewable raw materials. Last year we took the final step by introducing a cocoyl glutamate solution completely based on renewable raw materials without an organic solvent (AG 915 SF). This product fulfills every single aspect of the requirements mentioned in the introduction.
Additional benefits besides mildness can be provided by short-chain acyl derivatives (AG 8 and AG 11). The first of these is an acyl glutamate solution which has a fatty acid source (caprylic acid) of vegetable oil while the second is a solution of hydrolysed wheat protein linked to undecylenic acid which has a fatty acid source of castor oil. Both show a bacteriostatic and a mycostatic effect. AG 8 often is used in deodorising applications whereas AG 11 – inter alia – finds application in antidandruff formulations.
The amino acids used to prepare LS 9011 (based on lauric acid) and GC (based on coconut fatty acid) are sarcosine and glycine respectively. Despite being quite similar in the chemical structure there are some important differences between acyl sarcosinates and acyl glycinates in the physico-chemical properties in aqueous solutions. This is due to the ability of acyl glycinates to create intermolecular hydrogen bonds between the amide groups, a property that is lost when the amide hydrogen is replaced with an N-methyl group in acyl sarcosinates (Fig. 1). These attractive interactions between acyl glycinate molecules result – in combination with the absence of the bulky N-methyl groups – in a more dense and more stable ‘packing’ of acyl glycinates in comparison to acyl sarcosinates. For aqueous solutions this means a higher Krafft temperature of acyl glycinates.
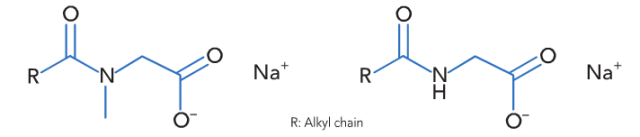
Figure.1 Acyl sarcosinate (left) and acyl glycinate (right). The hydrogen at the amide nitrogen enables acyl glycinates to create intermolecular hydrogen bondings.
The technical consequences are: Cocoyl glycinate is far less water-soluble and aqueous cocoyl glycinate solutions tend to be more viscous than the analogues with cocoyl sarcosinate. Our tests have shown that by mixing cocoyl glycinate and cocoyl sarcosinate there are no significant benefits confirming the experimental results published some years ago.1 In a completely different context we have recently proven the high efficiency of acyl glutamates as hydrotropic surfactants in neutral and alkaline formulations. Acyl glutamates are not only able to clear up cloudy formulations of nonionic surfactants effectively, but also able to reduce the viscosity of highly viscous concentrated anionic/nonionic surfactant mixtures drastically.2 We tested the capability of one of our acyl glutamates (AG 915 SF) as hydrotropic surfactants in highly viscous aqueous cocoyl glycinate solutions. Actually we succeeded in lowering the viscosity significantly. Besides a pure aqueous cocoyl glycinate solution (GC) – we developed a low viscous and easily thickenable aqueous cocoyl glycinate solution comprising cocoyl glutamate (GG).
LS 9011, GC and GG are applied as very mild co-surfactants for the preparation of hair shampoos and bath formulations. These products improve foam properties and work even in the presence of oils and emollients in high concentration. GC and GG are able to produce highly stable foam. This is in agreement with results published recently.3 LS 9011, GC and GG are completely based on the natural building blocks fatty acids and amino acids and contain neither solvents nor preservatives.
Enzymatically all representatives of this series can be metabolised by peptidases into fatty acids and amino acids. Both are essential for keeping the skin healthy: Free fatty acids are an important component of the skin barrier, whereas amino acids are part of the natural moisturising factor (NMF).
In contrast to the aqueous surfactant solutions of the Protelan-Series the Sebumol-Products are water-free surfactants or surfactant mixtures. The surfactants of the Protelan-Series are charged and water-soluble (depending on the pH), while most surfactants of the Sebumol-Series are uncharged and insoluble in water.
Products of the Sebumol-Series are esters but of different characteristics: PCA-Types link fatty acid derivatives with amino acid derivatives, PG-Types combine fatty acids with glycerol derivatives.
PCA-Types are prepared via a two-step reaction. Glutamic acid (biotechnologically produced) is cyclised to pyroglutamic acid (pyrrolidone carboxylic acid (PCA)) and esterified with a fatty alcohol (PCA esters, Fig. 2). LPC, SPC and ODPC are derivatives with lauryl alcohol, isostearyl alcohol (made from oleic acid) and octyldodecanol (made from two molecules decanol) respectively.
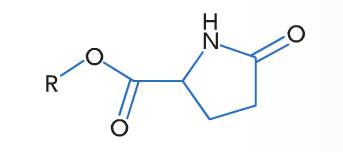
Figure 2. Sebumol PCA ester.
Those fatty alcohols are derivatives of natural fatty acids, therefore, the PCA esters are completely based on renewable raw materials. The PCA esters have 100% active matter. SPC and ODPC are clear colourless to yellowish liquids. At room temperature LPC is a soft solid wax with a melting point around 35˚C. It is therefore recommended for lip stick and lip gloss formulations in order to reduce the tackiness and to increase the spreading properties.
Moisturising effects of oil soluble substances seem to be contradictory. The PCA esters show that this is possible. They are oil soluble but can be cleaved enzymatically by lipases into PCA (which is part of the natural moisturising factor) and fatty alcohols (that provide emollient effects on the skin). Therefore, the PCA esters combine moisturising properties with emollient attributes in a single molecule.
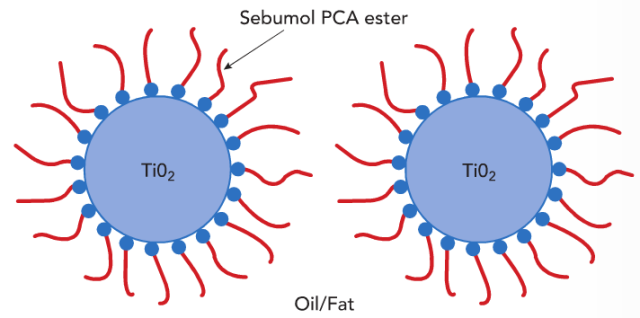
Figure 3. Hydrophobic ‘coating’ by alkyl chains prevents agglomeration.
PCA esters have a strong tendency to adsorb at the surface of inorganic pigments like titanium dioxide which are often used in personal care applications like colour cosmetics and sun protection. In lipophilic formulations with high content of fats or oils the PCA esters provide stable dispersions due to the alkyl chains (Fig. 3). This property is useful to enhance the performance of colour cosmetics. In particular the spreadability is improved, leading to an equal surface covering and an increased light reflection for more brilliancy (Fig. 4). Also the production process for colour cosmetic products is supported by using PCA esters: Less energy is needed to disperse the pigments. Additionally, the pigments are more evenly distributed in the product (Fig. 5).

Figure.4 “Equal surface covering’ and ‘more brilliancy’.
PG-Types are prepared by a two-step synthesis route. Glycerol from vegetable origin is condensated to polyglycerol (PG) which is esterified with fatty acids (polyglycerol esters). The PG-Types are polyglycerol ester blends combined in an intelligent way to get powerful liquid PEG-free emulsifiers. The advantages of using emulsifier blends instead of single emulsifiers are demonstrated in Figure 6 for a W/O emulsion

Figure 5. Creamy eye shadow prepared without (left) and with (right) Sebumol ODPC.
WO 200 and WO 300 are water-in-oil emulsifiers. WO 200 (Polyglyceryl-3 Diisostearate, Polyglyceryl-3 Dilaurate, Sodium Stearoyl Lactylate) is a special development mainly for synthetic oils. This emulsifier is a perfect choice for light yet even still rich W/O formulations like creams, lotions and sunscreens with inorganic and organic filters. WO 300 (Polyglyceryl-3 Polyricinoleate, Polyglyceryl4 Oleate) is the appropriate alternative for more polar oil mixtures including vegetable oils. This ingredient allows the formulator to create more nourishing W/O emulsions e.g. baby care products and products for sensitive skin.
To switch a water-in-oil emulsifier into an oil-in-water emulsifier we added as much anionic surfactant to the polyglycerol ester blends as necessary. B 1000 (Polyglyceryl-3 Polyricinoleate, Polyglyceryl3 Diisostearate, Sulfated castor oil, Polyglyceryl-3 Caprate) and S 1000 (MIPA-Cocoyl Sarcosinate, Polyglyceryl-3 Polyricinoleate, Polyglyceryl-3 Diisostearate, Polyglyceryl-3 Caprate) are oil-in-water emulsifiers for special applications like bath oils and shower oils.
Blooming bath oils provide a particular sensual experience caressing the soul whereas the oil is caressing the skin. When pouring blooming bath oils slowly in water they do not float on top as an oily layer. In a self-emulsifying process a milky white O/W-emulsion is formed (‘blooming effect’, Figure 7). The emulsified oils are able to nourish the skin. For this special product group we developed B 1000 which can easily be mixed with oils of different polarity to create PEG-free clear bath oils.
For PEG-free and clear shower oils we introduced S 1000 which provides creamy foam. S 1000 is miscible with high amounts of vegetable and synthetic oils.
An important other application for this product is in PEG-free clear facial cleansing oils. The corresponding facial cleansing oil solubilises and disperses (mediated by S 1000) makeup and excess sebum to provide a soft but thorough cleansing effect. After the application on the skin the product can easily be washed-off with water. This is due to the emulsifying power of S 1000 creating a thorough, rinseable cleansing emulsion.
The surfactants of the PG-Types are completely based on renewable raw materials (exception: Cocoyl sarcosinate in S 1000).
Cocamidopropyl betaines are also important ingredients in personal care products but are not part of this article as one of their components (dimethylaminopropylamine) is artificial: Despite the commonly used INCI desription Cocamidopropyl Betaine, there are products on the market that are made on the basis of palm kernel oil. As the fatty acid composition of coconut oil and the one of the cheaper but less sustainable (‘green’) palm kernel oil are similar, this is tempting. The corresponding Zschimmer & Schwarz products (Amphotensid B5 and Amphotensid B4 SB) are exclusively made from coconut oil.
All surfactants of the Protelan- and Sebumol-Series are completely based on natural building blocks (fatty acids, amino acids and glycerol) and their derivatives and are easily biodegradable. Besides the sarcosinate- and glycinate-derivatives (Protelan LS 9011, Protelan GC, Protelan GG, one ingredient in Sebumol S 1000) the surfactants of all Protelan- and Sebumol-Types are completely based on renewable raw materials.
Protelan AG 915 SF is a solvent-free product that fulfills all facets of the requirement profile mentioned in the introduction. All components are part of the human metabolism. The fatty acids and the glutamic acid are completely based on renewable raw materials and the product is easily biodegradable.
1 Bordes R, Tropsch J, Holmberg K. Role of an amide bond for self-assembly of surfactants. Langmuir 2010, 26 (5): 3077-83.
2 Wagner A. Lecture: New multifunctional hydrotropic surfactants. The 62nd SEPAWA Congress 2015.
3 Raykundaliya N, Bordes R, Holmberg K, Wu J, Somasundaran P, Shah DO. The effect on solution properties of replacing a hydrogen atom with a methyl group in a surfactant. Tenside Surfactants Detergents 2015; 52 (5): 369-74.